|

- 现金
- 222032 元
- 精华
- 285
- 帖子
- 67620
- 注册时间
- 2001-11-10
- 最后登录
- 2023-5-7






















|
37楼
 发表于 2005-12-24 05:36
发表于 2005-12-24 05:36
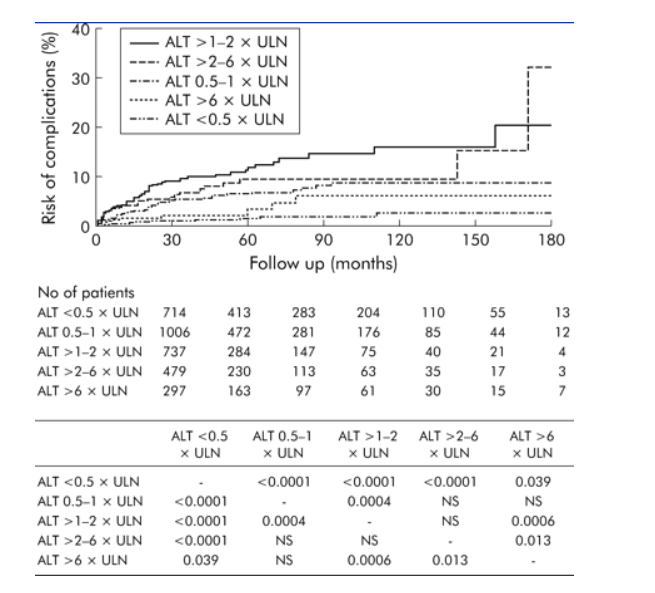
Figure 2. Cumulative risk of development of complications stratified according to alanine aminotransferase (ALT) levels on presentation. The table depicts p values for comparisons between different groups of patients. ULN, upper limit of normal.
DISCUSSION
The present study was limited by the relatively short period of follow up even though 307 patients (10.0%) were followed up for more than 10 years. Another limitation of the study was the absence of histological assessment. However, the end points of our study, the development of cirrhosis related complications and HCC, are of great clinical and prognostic relevance.
Several findings from the present study have direct implications for criteria for the treatment of CHB in the future. Firstly, the risk of complications increased as ALT levels on presentation increased from >0.5x ULN to 2x ULN. In contrast, patients with ALT levels above 6x ULN had a significantly lower risk for the development of complications (fig 2). These findings were confirmed using median ALT levels of patients during subsequent follow up. Acute exacerbations and high ALT levels (for example, >2x ULN) probably signify acute injuries to the liver which may not lead to permanent damage. This is probably analogous to the situation in acute viral hepatitis. However, in patients with only mild elevation of ALT, including those with ALT levels in the upper range of normal, the immune attack on the liver might be more insidious and chronic, leading eventually to more severe and permanent damage.
In the study of Kim et al showing an increased risk of mortality from liver disease in patients with ALT levels in the upper range of normal, it was suggested that the normal range of serum aminotransferase concentrations should be lowered in populations in which liver disease are common. 8 Even though the authors did not test their subjects for hepatitis B or C markers, their findings confirm ours. The present guidelines for treatment suggest that only patients with ALT levels >2x ULN should be treated.5-7 This would exclude patients with the highest risks for the development of complications from treatment.
Secondly, in the Asian population, disease activity continues to progress in a proportion of patients after HBeAg seroconversion. Median age for the development of complications in our patients was 57.2 years (table 2). Median age of HBeAg seroconversion was 35 years. More than two thirds of the patients were already anti-HBe positive when they developed complications. The cumulative risk for the development of complications was comparable between patients positive for HBeAg and for anti-HBe on presentation. A Taiwan study by Yang et al. claimed that HBeAg positivity was associated with an increased risk of HCC.12 HBeAg status of the patients in this study however was checked only at the time of enrolment whereas the development of HCC was observed during the subsequent 10 years of follow up. HBeAg/anti-HBe status at the time of development of HCC was not assessed. This study therefore can provide no clue as to the HBeAg status of patients at the time of HCC development. Another smaller Taiwan study found that although HBeAg seroconversion confers a favourable outcome in some patients, active hepatitis can occur after HBeAg seroconversion, leading to cirrhosis and HCC.13 McMahon et al, in their study, found that "seroconversion from HBeAg to anti-HBe, and even loss of hepatitis B surface antigen, did not protect patients from development of hepatocellular carcinoma".14 These latter two studies confirm the findings of the present study, that the majority of cirrhosis related complications and HCC develop after HBeAg seroconversion. The onset of cirrhosis occurs during the prolonged process of HBeAg seroconversion in Asian patients.15 Cirrhosis will continue to worsen or develop after HBeAg seroconversion.16-18 Thus HBeAg seroconversion should only be regarded as a step towards viral suppression, and therapy may need to be continued after HBeAg seroconversion.
Thirdly, progression of cirrhosis is more likely to be related to the low level of viraemia in a large proportion of patients who are anti-HBe positive. In the current study, only 22.8% of anti-HBe patients had undetectable HBV DNA levels by the PCR based assay; 32.3% had more than 105 copies/ml. (That the wild-type virus is as likely to cause complications as the precore mutants have been analysed in a separate study19.)
Among the 56% of patients with complications in whom HBV DNA levels were undetectable by the Digene Hybrid Capture assay, over 70% had HBV DNA levels detectable by the Amplicor HBV Monitor test. Twenty nine per cent of patients had undetectable HBV DNA (that is, <200 copies/ml). This is evidence against the proposal that disease progression is unlikely once HBV DNA levels become less than 105 copies/ml.20 Our findings support the conclusion of Chu and colleagues21 that there is no cut off HBV DNA value for differentiating active from inactive disease in HBeAg negative patients. This implies that prolonged and maximal suppression of HBV DNA to levels below the detection limit of PCR based assays may be necessary to reduce the risk of complications.
In conclusion, prolonged low level viraemia causing insidious and continual liver damage, as reflected by relatively mild elevations in ALT levels, is the most likely pathway leading to the development of complications for Asian patients with CHB. Long term antiviral therapy aiming at maximal suppression of HBV even after HBeAg seroconversion may be required for Asian patients.
MORE RESULTS
Demographics
A total of 3233 HBV patients were recruited. Demographic data on presentation are shown in table 1. Median and mean duration of follow up were 29 (range 6-291) months and 46.9 (SD 46.6) months, respectively.
HBeAg seroconversion
Among 1274 patients positive for HBeAg on presentation, 512 patients (40.2%) had HBeAg seroconversion at subsequent follow up. Median age at HBeAg seroconversion was 35 years (range 3.6-77.4).
Development of complications
A total of 170 patients (5.3%) developed at least one complication. The number of patients developing each complication was as follows: ascites 96 (3.0%) with 30 patients (0.9%) also having SBP, oesophageal varices 59 (1.8%), encephalopathy 40 (1.2%), and HCC 95 (2.9%). Of the 59 patients with oesophageal varices, 15 had clinical bleeding; the rest were detected by endoscopy. The cumulative risk for the development of complications is shown in fig 1. By the end of 10 years and 15 years of follow up, 8% and 12% of patients, respectively, had developed complications.
Median age and HBeAg/anti-HBe status at the time of development of each complication are shown in table 2; 73.5% of patients were positive for anti-HBe when complications developed. As this might be related to the higher prevalence of anti-HBe positivity in the age groups when complications occurred, patients were stratified according to age. For patients under 50 years of age, the prevalence of anti-HBe positivity for patients who developed complications was significantly higher compared with that of patients with no complications (73.1% (38/52) v 56.4% (1463/2596), respectively; p = 0.016). For patients over 50 years of age, there was no significant difference in anti-HBe positivity between patients who developed complications and patients with no complications (73.7% (87/118) v 80.1% (374/467), respectively; NS).
Factors associated with the development of complications
Demographics on presentation
ALT & HBV DNA discussed above.
Males had a higher cumulative risk for the development of complications than females (p<0.0001). Patients who developed complications, compared with patients without complications, were older (median age 55 years (range 19-82) v 38 years (range 1-85); p<0.0001), had a higher chance of the presence of stigmata of chronic liver disease (p<0.0001), and were more likely to present with hepatitis symptoms (p<0.0001).
Liver biochemistry and AFP
Patients who developed complications had significantly lower median albumin levels, higher median ALT levels, higher median bilirubin levels, and higher median AFP levels on presentation compared with patients without complications (table 3). However, median bilirubin and AFP levels for those who developed complications were still within normal limits.
HBeAg status on presentation
There was no significant difference in the cumulative risk for the development of complications between HBeAg positive patients and anti-HBe positive patients (p = 0.12). This was also true when each type of complication was analysed separately.
Effect of exacerbation
The effect of exacerbation on the development of complications was analysed with respect to the number of episodes of exacerbation, duration of exacerbation, peak ALT levels, and peak AFP levels. The only significant risk factor was peak AFP levels of 100 ng/ml or more during or after an exacerbation (p = 0.0001 compared with patients with AFP level less than 100 ng/ml).
Independent factors associated with development of complications and poor survival
Using the Cox proportional hazards model, male sex (p<0.0001), presence of stigmata of chronic liver disease (p = 0.045), increasing age (p<0.0001), low albumin level on presentation (p<0.0001), and high AFP level on presentation (p = 0.001) were found to be independent factors associated with a higher cumulative risk for the development of complications.
For survival analysis, male sex (p = 0.004), presence of hepatitis symptoms (p = 0.02), increasing age (p<0.0001), and low albumin level on presentation (p<0.0001) were found to be independent factors associated with a shorter actuarial survival.
PATIENTS AND METHODS
All Chinese CHB patients who were followed up in the Hepatitis Clinic, Department of Medicine, Queen Mary Hospital, Hong Kong, during the period from January 1976 to December 2000 were recruited. A total of 346 patients who already had cirrhosis related complications including ascites, spontaneous bacterial peritonitis (SBP), oesophageal varices, encephalopathy, or HCC (collectively termed as "complications") on presentation were excluded from the study. Patients with hepatitis C (n = 25) and hepatitis D (n = 10) coinfection, a history of significant alcohol consumption (n = 19), evidence of coexisting autoimmune hepatitis (n = 4), Wilson’s disease (n = 5), or primary biliary cirrhosis (n = 5) were also excluded. In addition, 494 patients who received interferon, lamivudine, or other investigational modalities for the treatment of CHB were excluded because these would not represent the natural history of the disease. The long term outcome of interferon treated patients has been analysed and published separately.11 All of the studies of interferon included patients with normal as well as elevated ALT levels. Their exclusion should not bias the outcome of our current analysis.
All patients were positive for hepatitis B surface antigen by micro-particle enzyme immunoassay (MEIA; Abbott Laboratories, Chicago, Illinois, USA) for at least six months. HBeAg and antibodies to HBeAg (anti-HBe) (ELISA; Abbott Laboratories), liver biochemistry, and fetoprotein (AFP) were checked every 3-6 months. Continual clinical assessments, including the development of ascites, were carried out during each follow up. Patients with significant symptoms or abnormalities in blood tests were recalled and, if necessary, admitted to hospital. HBV DNA levels were measured by the Digene Hybrid Capture assay (Digene Corporation, Gaithersburg, Maryland, USA; lower limit of detection 140 000 copies/ml). In order to determine HBV DNA levels at relatively low titre, HBV DNA levels of all anti-HBe positive patients were determined again using a polymerase chain reaction (PCR) based assay (Cobas Amplitor HBV Monitor Test; Roche Diagnostics, Branchburg, New Jersey; lower limit of detection 200 copies/ml).
Patients with ALT levels that were increased to 1.5 times the upper limit of normal (ULN) (upper limit: 53 and 31 U/l for males and females, respectively) were defined as having exacerbation of chronic HBV infection if other common causes of ALT elevation were excluded, including other viral hepatitis, drug induced hepatitis, alcoholic hepatitis, and steatohepatitis. Peak ALT, peak bilirubin, peak AFP levels, and duration for each exacerbation were noted.
HBeAg seroconversion was defined as loss of HBeAg with development of anti-HBe on at least two consecutive follow up visits.
Ultrasonogram was arranged for patients with elevated AFP (>20 ng/ml) if ALT was normal and for patients with persistently elevated AFP even if ALT was raised. Computer tomography and/or hepatic angiogram was performed if ultrasound showed suspicion of HCC. For patients with clinical detection of ascites, splenomegaly, or persistently low albumin of <35 g/l, a screening oesophageogastroduodenoscopy was performed for detection of oesophageal varices. The occurrence of complications and survival time were recorded.
Statistical analysis
All statistical analyses were performed using the Statistical Program for Social Sciences (SPSS 10.0 for Windows; SPSS Inc., Chicago, Illinois, USA). Normality of the distribution of continuous variables was tested by the Komolgorov-Smirnov test. The Mann-Whitney U test was used for continuous variables with skewed distribution and the 2 test with Yates’ correction factor or Fisher’s exact test was applied for categorical variables. Differences in paired parameters were tested by the Wilcoxon signed ranks test. The Kaplan-Meier method using the log rank test was applied for calculation of the cumulative risk of development of complications and survival. The Cox hazard proportional model was used to test the associations between different variables and the development of complications and survival. |
|
| | |
|
 发表于 2005-12-23 11:29
发表于 2005-12-23 11:29
 发表于 2005-12-23 21:05
发表于 2005-12-23 21:05
 发表于 2005-12-24 04:06
发表于 2005-12-24 04:06

 发表于 2005-12-24 04:53
发表于 2005-12-24 04:53

 发表于 2005-12-24 05:29
发表于 2005-12-24 05:29

 发表于 2005-12-24 05:34
发表于 2005-12-24 05:34

 发表于 2005-12-24 05:36
发表于 2005-12-24 05:36

 发表于 2005-12-24 07:19
发表于 2005-12-24 07:19
 发表于 2005-12-25 01:58
发表于 2005-12-25 01:58
 发表于 2005-12-26 00:42
发表于 2005-12-26 00:42