The percentage of patients with HBV DNA of less than 400 c/mL were 45 percent, 63 percent, 56 percent, and 71 percent for the pradefovir 5, 10, 20, and 30 mg (QD) groups, respectively, and 36 percent for the Hepsera group.
No patient demonstrated an increase in serum creatinine levels over baseline of greater than or equal to 0.5 mg/dL, a marker for the severe renal toxicity associated with higher doses of adefovir. Renal safety was comparable between all treatment groups. There were no serious adverse events related to treatment. The most frequently reported adverse events were similar across all treatment groups, including Hepsera. No dose-related trends regarding safety were identified and no events resulted in a patient being withdrawn prematurely from treatment.
Paul Laikind Ph.D., chairman, president and chief executive officer at Metabasis, said, "The results reported by Valeant today give us greater confidence that this drug could become a leading product for the treatment of hepatitis B, should it be approved. The combination of robust efficacy, even in difficult to treat patients that have failed on previous treatment regimens, good safety and the expected low viral resistance appears to provide potential advantages over competitive products. The team at Valeant has done an excellent job with the product, and we look forward to their initiation of Phase 3 trials later this year."
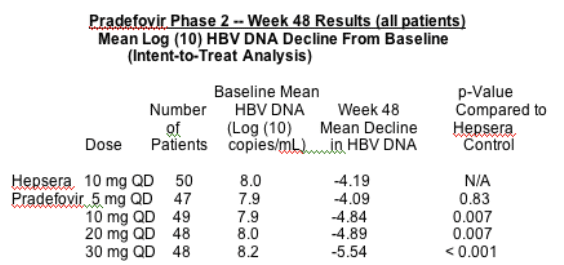
Mark Erion, Ph.D., executive vice president, research and development and chief scientific officer, said, "The greater than 5.5 log reduction of HBV DNA level in the 30 mg pradefovir cohort from a baseline of only around 8 logs is impressive, as is the fact that 71 percent of patients at that dose achieved undetectable levels of HBV DNA compared to only 36 percent of patients treated with Hepsera. This strong efficacy combined with the good safety data suggests that our HepDirect technology is performing as expected."
Valeant plans to present the detailed Phase 2 results at the 41st Annual Meeting of the European Association for the Study of the Liver in April 2006 in Vienna, Austria. Phase 3 pivotal trials for pradefovir are expected to begin in mid-2006.
About Hepatitis B
Hepatitis B is a potentially fatal disease that can lead to complications such as cirrhosis and liver cancer. Approximately 2 billion people worldwide are estimated to have hepatitis B, with 350-400 million people estimated to be chronically infected. According to a recent study, the HBV market currently represents more than $1 billion in annual sales, and is expected to grow to over $2.8 billion by 2012.
Pradefovir is an investigational compound that has not been found by the FDA or any other regulatory agency to be safe or effective in the diagnosis, mitigation, treatment or cure of any disease or illness. It may not be sold or promoted in the United States unless and until the FDA has approved its New Drug Application. Similar restrictions apply in other countries.
About Metabasis (www.mbasis.com):
Metabasis Therapeutics, Inc. is a biopharmaceutical company uniquely focused on the discovery, development and commercialization of novel drugs to address some of the world's most widespread and costly chronic diseases involving pathways in the liver. The Company has established a pipeline that includes clinical stage and preclinical product candidates targeting large markets with significant unmet medical needs. Targeted diseases include major metabolic diseases such as diabetes, hyperlipidemia and obesity as well as liver diseases such as hepatitis and primary liver cancer. Metabasis has developed several proprietary technologies for use in discovering and optimizing drugs, including the NuMimetic(TM) and HepDirect(TM) technologies. Metabasis is continuing to identify and develop new product candidates using its proprietary technologies and expertise.
Forward-Looking Statements:
Statements in this press release that are not strictly historical in nature constitute "forward-looking statements." Such statements include, but are not limited to, references to the design, efficacy, safety, use and potential further development and clinical trials of pradefovir, the potential of pradefovir in the market, and the potential and progress of, Metabasis' other clinical and preclinical compounds. Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause Metabasis' actual results to be materially different from historical results or from any results expressed or implied by such forward-looking statements. These factors include, but are not limited to, risks and uncertainties related to Metabasis' dependence on Valeant and its other licensees or collaborators for the clinical development and registration of certain of its product candidates, among other things; the initiation, progress and timing of clinical trials for pradefovir and Metabasis' other product candidates; the ability to duplicate results from early stage clinical trials in later stage clinical trials; serious adverse side effects or inadequate efficacy of, or serious adverse events related to, Metabasis' product candidates or proprietary technologies; difficulties or delays in development, testing, obtaining regulatory approval, producing and marketing Metabasis' product candidates; the potential and progress of preclinical compounds and programs; Metabasis' ability to retain and motivate key management and scientific personnel; and other factors discussed in the "Risk Factors" section of Metabasis' Quarterly Report on Form 10-Q for the quarter ended September 30, 2005. All forward-looking statements are qualified in their entirety by this cautionary statement. Metabasis is providing this information as of this date of this release and does not undertake any obligation to update any forward-looking statements contained in this release as a result of new information, future events or otherwise.
CONTACT: Paul Laikind, Ph.D., Chairman, CEO & President of
MetabasisTherapeutics, Inc., +1-858-622-5550 |
 发表于 2006-4-7 02:25
发表于 2006-4-7 02:25
 发表于 2006-4-7 02:25
发表于 2006-4-7 02:25
 发表于 2006-4-7 21:06
发表于 2006-4-7 21:06
 发表于 2006-4-12 02:56
发表于 2006-4-12 02:56
 发表于 2006-4-21 09:12
发表于 2006-4-21 09:12