Entecavir 24 Week Followup Off-Treatment in HBeAg+
“Entecavir Leads to Sustained Response Off-Treatment in Nucleoside-Naïve, HBeAg(+) Patients Who Met On-Treatment Response Endpoints at 48 Weeks of Therapy in Phase III Study ETV-022”
Reported by Jules Levin
Robert Gish presented this followup data & information at DDW’s HBV oral session.
INTRODUCTION
A primary goal of therapy for HBeAg+ chronic hepatitis B is to reduce HBV DNA to undetectable levels & promote loss of HBeAg & HBeAg seroconversion in order to promote the following:
--ALT normalization
--improvement in liver histology
--reduce risk of liver disease progression
(note from Jules Levin: a number of studies presented at EASL (April 2005) & at DDW show that reducing HBV DNA to <300 copies/ml & to sustain this appears to provide the best way to prevent liver disease progression to cirrhosis & liver cancer.)
Entecavir (ETV) is a potent & selective inhibitor of HBV polymerase. ETV has demonstrated superiority to lamivudine (3TC), during 48 weeks treatment, in three Phase III clinical trials involving:
--nucleoside-naïve HBeAg(+) patients: Study ETV-022
--nucleoside-naïve HBeAg(-) patients: Study ETV-027
--lamivudine (LAM) refractory HBeAg(+) patients: Study ETV-026
ETV has a clinical safety profile comparable to LAM.
STUDY OBJECTIVE
The objective of this analysis is to assess the 24-week off-treatment sustained response rates in HBeAg(+), nucleoside-naïve patients who achieved protocol defined complete response to ETV or LAM therapy by week 48.
Patients received ETV 0.5 mg once daily (n=354) or LAM 100 mg once daily (n=355). Following is a picture of the Study design.
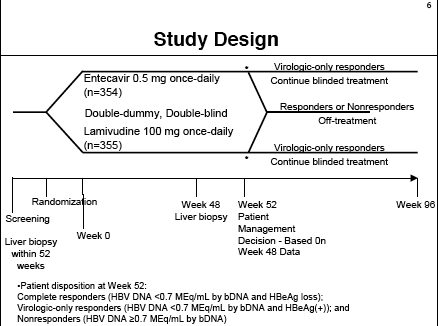
INCLUSION CRITERIA (ETV-022)
>16 yrs old
evidence of chronic hepatitis by liver biopsy during screening or within 52 weeks prior to randomization.
HBeAg(+)
<12 weeks prior nucleoside/nucleotide treatment.
Last dose of any anti-HBV therapy >24 weeks prior to randomization.
HBV DNA >=3 MEq/mL at screening.
Serum ALT >=1.3xULN
Compensated liver function
No coinfection with HIV, HCV or HDV
BASELINE DISEASE CHARACTERISTICS
Mean HBV DNA by PCR: 9.62-9.69 log
ALT (mean) IU/L: 140-146
Genotype A: 27%; B: 19-22%; C: 25-31%; D; 10-14%; F; 3-6%
DEMOGRAPHICS
Males: 75%
Age: 35 yrs
40% Caucasian; 57% asian’ 3% other
PROTOCOL DEFINED COMPLETE VIROLOGIC RESPONSE, patient cohort for this analysis: HBV DNA <0.7 MEq/mL by bDNA & loss of HBeAg at week 48.
WEEK 48 ENDPOINT
RESPONSE (HBV DNA <0.7 MEq/mL by bDNA & loss of HBeAg):
21% for ETV (n=74); 19% for LAM (n=67).
VIROLOGIC ONLY RESPONSE
HBV DNA <0.7 MEq/mL by bDNA but positive for HBeAg
70% for ETV, 46% LAM
NON-RESPONSE
HBV DNA >=0.7 MEq/mL by bDNA
5% for ETV
26% for LAM
ENDPOINTS FOR THIS ANALYSIS
Sustained response outcomes at 24 weeks off-treatment assessed as:
--ALT normalization (ALT <1.25xULN)
--response (HBV DNA <0.7 MEq/mL by bDNA & loss of HBeAg)
--combined response & ALT normalization
This included: percentage efficacy outcomes & Kaplan-Meier proportion of patients remaining in responder category.
SUMMARY OF RESULTS at 24-WEEK OFF-TREATMENT FOLLOW-UP
ALT NORMALIZATION (ALT<1.25xULN):
76% ETV
58% LAM
RESPONSE (HBV DNA <0.7 MEq/mL & HBeAg loss):
82% ETV
73% LAM
RESPONSE & ALT NORMALIZATION:
73% ETV n=74
57% LAM n=67
A POTENTIAL PREDICTOR of OFF-TREATMENT RELAPSE
Among the 11 ETV patients who lost their response off-treatment: --8 were late responders (week 36 or later) --3 were early responders (week 24 or earlier)
Among the 15 LAM patients who lost their response off-treatment:
--8 were late responders (week 36 or later)
--7 were early responders (week 24 or earlier)
AUTHOR’s CONCLUSIONS:
The sustained response with ETV was durable after only 48 weeks of treatment in the majority of patients (82%) who met the treatment response definition & was numerically greater than LAM.
In addition:
--patients treated with ETV who lost vresponse during off-treatment follow-up were more likely to achieve the protocol definition of response in later weeks of therapy.
--This suggests that complete virologic response, defined as HBV DNA <0.7 MEq/mL & loss of HBeAg, may be a strong predictor of sustained off-treatment response to ETV.
|  发表于 2005-5-31 03:34
发表于 2005-5-31 03:34
 发表于 2005-5-31 11:44
发表于 2005-5-31 11:44