|
 
- 现金
- 62111 元
- 精华
- 26
- 帖子
- 30437
- 注册时间
- 2009-10-5
- 最后登录
- 2022-12-28

|
Efficacy and Safety Results of the Phase 2 JNJ-56136379 JADE Study in Patients with Chronic Hepatitis B: Interim Week 24 Data
| | | | | EASL: Short-term treatment with RNA interference therapy, JNJ-3989, results in sustained hepatitis B surface antigen suppression in patients with chronic hepatitis B receiving nucleos(t)ide analogue treatment (08/27/20)
EASL 2020 Aug 27-29 virtual
Reported by Jules Levin
Harry L.A. Janssen1, Jinlin Hou2, Tarik Asselah3, Henry L.Y. Chan4, Fabien Zoulim5, Yasuhito Tanaka6, Ewa Janczewska7, Ronald Nahass8, Stefan Bourgeois9, Maria Buti10, Pietro Lampertico11, Oliver Lenz12, Thierry Verbinnen12, Joris Vandenbossche12, Willem Talloen12, Michael Biermer12, Ronald Kalmeijer13, Maria Beumont13, Umesh Shukla13
1Toronto General Hospital, Toronto, ON, Canada; 2Nanfang Hospital, Southern Medical University, Guangzhou, China; 3University Paris Diderot, INSERM UMR1149, Hôpital Beaujon, Clichy, France; 4The Chinese University of Hong Kong, Hong Kong SAR, China; 5Hospices Civils de Lyon and Lyon University & INSERM U1052-Cancer Research Institute of Lyon, Lyon, France; 6Nagoya City University Graduate School
of Medical Sciences, Nagoya, Japan; 7University of Silesia, School of Health Sciences in Bytom, Medical University of Silesia, Poland; 8ID Care, Hillsborough, NJ, USA; 9ZNA Jan Palfijn, CPU, Antwerp, Belgium; 10Hospital Universitario Vall d'Hebrón and CIBERHED del Instituto Carlos III, Barcelona, Spain; 11CRC "A. M. and A. Migliavacca" Center for Liver Disease, Division of Gastroenterology and Hepatology, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Università degli Studi di Milano, Milan, Italy; 12Janssen Pharmaceutica NV, Beerse, Belgium; 13Janssen Pharmaceuticals R&D, Titusville, NJ, USA 
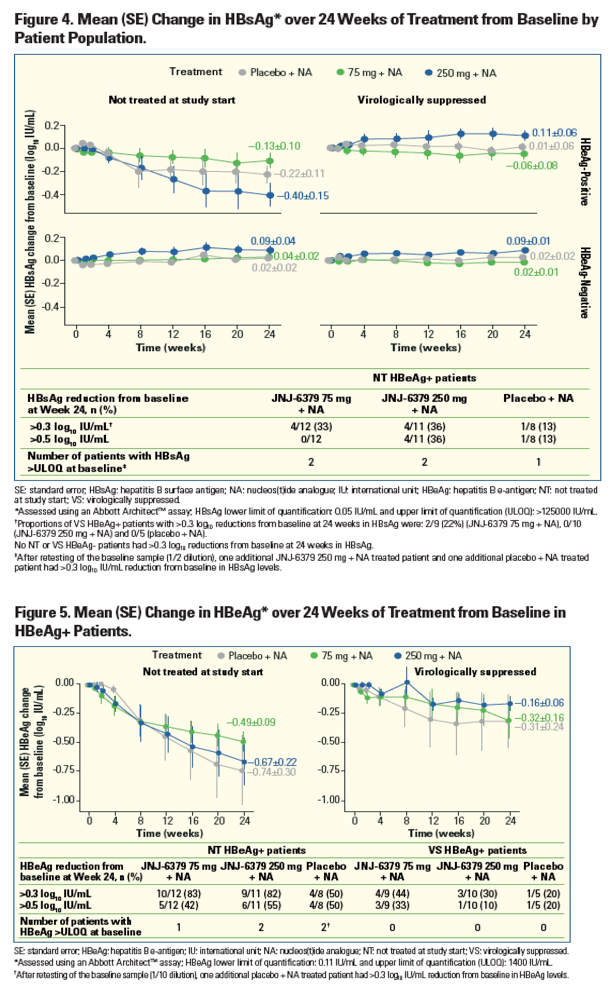
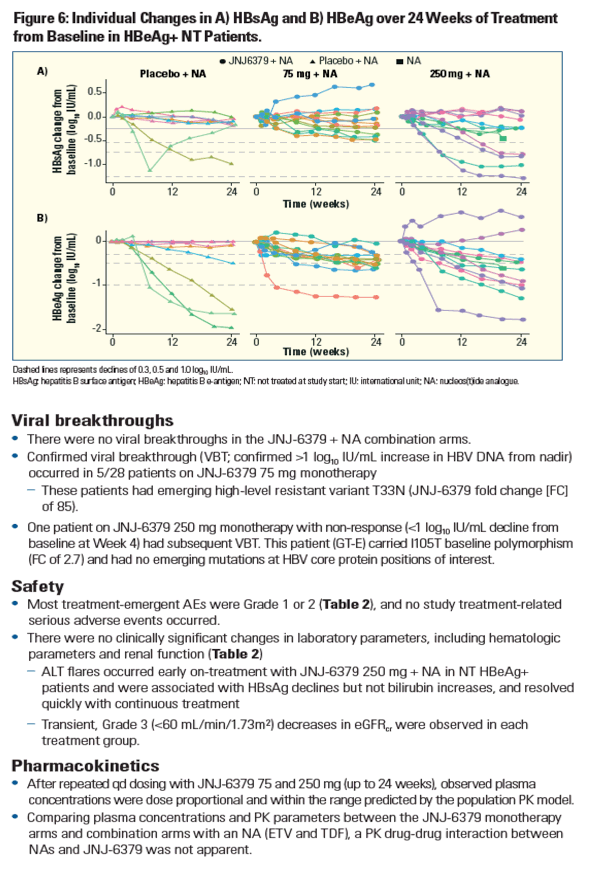
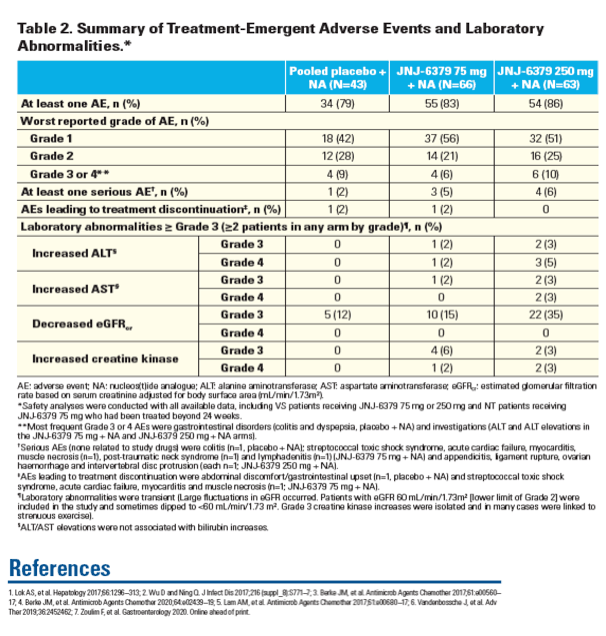
| | |
|  |  |
|
| | |
|
|