HBsAg Kinetics in Retreatment Decision for Off-Therapy Hepatitis B Flare in HBeAg-Negative Patients [url=]Yun-Fan Liaw[/url]
, [url=]Wen-Juei Jeng[/url]
, [url=]Ming-Ling Chang[/url]
Liver Research Unit, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taipei, Taiwan
DOI: https://doi.org/10.1053/j.gastro.2018.03.066 | 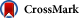
 Article Info Article Info
Dear Editors:A high rate of hepatitis B surface antigen (HBsAg) seroclearance after cessation of nucleos(t)ide analogue therapy has been reported previously in patients with hepatitis B e antigen (HBeAg)-negative chronic hepatitis B.[url=]1[/url], [url=]2[/url], [url=]3[/url] More important, patients who were not retreated for clinical relapses off medication had a much higher HBsAg seroclearance rate than those who received retreatment.[url=]1[/url], [url=]3[/url] These findings have raised an important issue of how to differentiate a benign or beneficial alanine aminotransferase (ALT) flare that retreatment can be withheld from a detrimental one that requires retreatment.[url=]2[/url], [url=]3[/url] It is, therefore, crucial to define such a “stop-and-watch” strategy and optimal timing for retreatment that is not too soon to allow sufficient immune clearance response but not too late to prevent adverse outcomes.[url=]3[/url] Hepatitis B flare, an event of ALT to >5× the upper limit of normal reflecting increasing endogenous immune response against hepatitis B virus (HBV), may be followed by sustained remission, persistent ALT elevation, or hepatic decompensation.[url=]4[/url] In patients with “effective immune clearance,” quantitative HBsAg levels surge before the ALT peak, and decrease along with ALT normalization to achieve remission. Conversely, quantitative HBsAg levels remain high or increase further in patients with “ineffective immune clearance”.[url=]4[/url] To test whether this combination of ALT and HBsAg kinetics can be used to make retreatment decisions for clinical relapse, we conducted a proof-of-concept case study using serial serum samples collected every 1 to 2 weeks from 2 patients treated with nucleos(t)ide analogue who were HBeAg-negative and had an off-therapy hepatitis flare of similar degree but with different outcomes. The results showed that, at the end of therapy, the quantitative HBsAg of patient A was 250 IU/mL, increased to 260 IU/mL upon virologic relapse, decreased to 146 IU/mL when ALT peaked at 356 U/L, followed by ALT normalization and a gradual quantitative HBsAg decrease to a level of 1.5 IU/mL, which is very close to HBsAg seroclearance (Figure 1A).
 Figure 1The clinical course in 2 hepatitis B e antigen (HBeAg)-negative patients with hepatitis B flare after end of antiviral therapy (EOT). (A) No retreatment is needed in patients with successive HBsAg decline, whereas (B) retreatment is required in patient with increasing HBsAg levels. The horizontal dotted line indicates the upper limit normal of alanine aminotransferase (ALT; 36 U/L). Figure 1The clinical course in 2 hepatitis B e antigen (HBeAg)-negative patients with hepatitis B flare after end of antiviral therapy (EOT). (A) No retreatment is needed in patients with successive HBsAg decline, whereas (B) retreatment is required in patient with increasing HBsAg levels. The horizontal dotted line indicates the upper limit normal of alanine aminotransferase (ALT; 36 U/L).
View Large Image | View Hi-Res Image | Download PowerPoint Slide
In contrast, the end of antiviral therapy quantitative HBsAg of patient B was 306 IU/mL, which decreased to 281 IU/mL at virologic relapse, surged to 5764 IU/mL when ALT flared to 247 U/L, followed by ALT fluctuation and increased quantitative HBsAg to 19,623 IU/mL when ALT increased to 238 U/L and HBV DNA to 4.42 × 107 IU/mL. He was then treated with a nucleos(t)ide analogue to prevent further possible deterioration (Figure 1B). In clinical studies, quantitative HBsAg levels were measured every 3 to 6 months or even yearly. So far, only a small study of 15 HBeAg-negative patients monitored quantitative HBsAg every 4 weeks in the first 12 weeks after cessation of nucleos(t)ide analogue treatment, but not during a clinical relapse.[url=]5[/url] As shown in Figure 1, some of the quantitative HBsAg changes would not have been detected if it were assayed every 1 to 3 months. The serial HBsAg changes were different between these 2 HBeAg-negative patients with off-nucleos(t)ide analogue clinical relapse and hepatitis flare. Because quantitative HBsAg has been considered as a surrogate marker of infected hepatocyte,[url=]6[/url] the successive and profound HBsAg decrease after hepatitis B flare in patient A reflects an “effective immune clearance,” whereas the increasing HBsAg levels in patient B suggests that his immune response was ineffective or failed to control the HBV. It could be anticipated that if patient B had remained untreated, his ongoing hepatitis may lead to adverse outcome(s). In conclusion, it seems appropriate to include quantitative HBsAg assay every 3 months in the off-nucleos(t)ide analogue monitoring plan and more frequently when ALT is increasing, at least before and after the peak of ALT. If HBsAg level is decreasing, retreatment can be withheld or is not necessary even in patients with hepatitis flare. In contrast, patients whose HBsAg is increasing and ALT remains elevated may need retreatment. Immediate retreatment is required if patients show signs of hepatic decompensation, such as increasing serum bilirubin and a prolonged prothrombin time. Further large studies are required to confirm the value of quantitative HBsAg monitoring in the proposed “stop-and-watch strategy,” which may increase HBsAg seroclearance rate.[url=]7[/url] |