|
 
- 现金
- 62111 元
- 精华
- 26
- 帖子
- 30437
- 注册时间
- 2009-10-5
- 最后登录
- 2022-12-28

|
| | Association of Nucleos(t)ide Analogue Therapy With Reduced Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis B-A Nationwide Cohort Study
| | | | | Download the PDF here
"In conclusion, nucleos(t)ide analogue use is associated with reduced risk of HCC in CHB patients. Age, sex, liver cirrhosis, and diabetes mellitus modify this association. More studies are needed to explore the wider use of nucleos(t)ide analogues for prolonged periods to decrease the incidences of HCC.Without controlling for other factors, nucleos(t)ide analogue treatment was associated with reduced risk of HCC development (hazard ratio [HR] = 0.34; 95% CI: 0.32-0.37; P < .001)[63% lower risk] ......After adjusting for competing mortality and other confounders, nucleos(t)ide analogue treatment was associated with a reduced risk of HCC, with an adjusted hazard ratio of 0.37 (95% CI, 0.34-0.39; P < .001). ....The treated cohort had a significantly lower incidence of HCC (n = 992 [4.6%]) when compared with the untreated cohort (n = 4454 [20.6%])"
"For noncirrhotic CHB patients, mean annual incidences of HCC were 0.68% and 2.97% for the treated and the untreated cohorts, respectively. Adjusted HR was 0.25 for the use of nucleos(t)ide analogues.......For cirrhotic CHB patients, the mean annual incidences of HCC in the present study were 3.90% and 4.94% for treated and untreated cohorts, respectively. The adjusted HR was 0.72 for the use of nucleos(t)ide analogue"
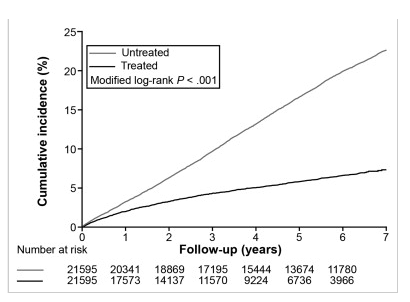
" ......The treated cohort had a significantly lower 7-year incidence of HCC (7.32%; 95% confidence interval [CI], 6.77%-7.87%) than controls (22.7%; 95% CI, 22.1%-23.3%; P < .001)......Competing mortality (death before development of HCC) was significantly lower in the treated cohort (n = 1036 [4.8%]) than in the untreated cohort (n = 2256 [11.8%]). Overall mortality in the treated cohort (n = 1406 [6.5%]) was also significantly lower than in the untreated cohort (n = 4778 [22.1%])."
"Older age, male sex, and liver cirrhosis (HR 1.92; highest risk) were found to be risk factors for increased HCC risk. Patients with comorbidities including liver decompensation, hypertension, chronic obstructive pulmonary disease, acute coronary syndrome, and cerebral vascular diseases were found to be associated with reduced risk of HCC due to higher competing mortality. Use of statin and use of nonsteroidal anti-inflammatory drugs or aspirin were associated with significantly lower risk of HCC in CHB patients (Table 2)."
Figure 2: Cumulative incidences of HCC after adjustment for competing mortality. Calculation and comparison of cumulative incidences in competing risk data ratios were conducted using modified Kaplan-Meier method and Gray's method. Patients who developed HCC during the first 3 months were excluded. Untreated, CHB patients not receiving nucleos(t)ide analogues; Treated, CHB patients receiving nucleos(t)ide analogues.
".....reimbursement for nucleos(t)ide analogues requires twice-elevated aminotransferase and higher HBV DNA levels (>2000 IU/mL), and reimbursement for hepatoprotective agents (control group) requires only elevated aminotransferase level (ALT ≥1x), the higher baseline HBV DNA levels and ALT levels in the treated cohort might have led to a more conservative estimation of the protective effect of nucleos(t)ide analogues."
-------------------
Association of Nucleos(t)ide Analogue Therapy With Reduced Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis B-A Nationwide Cohort Study
Gastroenterology
July 2014
Chun-Ying Wu,1,2,3,4 Jaw-Town Lin,5,6,7 Hsiu J. Ho,5 Chien-Wei Su,2,8,9 Teng-Yu Lee,1,3Shen-Yung Wang,8 Chuhui Wu,8 and Jaw-Ching Wu8,101Division of Gastroenterology, Taichung Veterans General Hospital, Taichung, Taiwan; 2Faculty of Medicine, School ofMedicine, National Yang-Ming University, Taipei, Taiwan; 3Department of Public Health and Graduate Institute of ClinicalMedicine, China Medical University, Taichung, Taiwan; 4Department of Life Sciences, National Chung-Hsing University,Taichung, Taiwan; 5School of Medicine, Fu Jen Catholic University, Taipei, Taiwan; 6Department of Internal Medicine,E-Da Hospital/I-Shou University, Kaohsiung, Taiwan; 7Institute of Population Health Sciences, National Health ResearchInstitutes, Miaoli, Taiwan; 8Institute of Clinical Medicine and Genomic Research Center, National Yang-Ming University, Taipei,Taiwan; 9Division of Gastroenterology, Taipei Veterans General Hospital, Taipei, Taiwan; and 10Translational Research Division,Medical Research Department, Taipei Veterans General Hospital, Taipei, Taiwan |
|
|