| Ancient origin of hepatitis B viruses revealed by DNA fossils in bird genomes The hepatitis B virus originally infected birds back when the dinosaurs still roamed the planet, according to a newly published study of genomic bird DNA, a finding that may help improve human health outcomes.
 Artistic interpretation of a hepatitis B virus fossil. Artistic interpretation of a hepatitis B virus fossil.
Image copyright: Alexander Suh & somersault18:24. doi:10.1038/ncomms2798.
 As the old adage goes; "One man's trash is another man's treasure": what has often been described as "junk DNA" has revealed a hidden gem. Not only can we find the ancient ancestor of the human hepatitis B virus nestled in songbird genomes, but according to research published recently by a team of scientists at the University of Münster, this virus is 63 million years older than originally thought, a finding that may help improve human health outcomes. As the old adage goes; "One man's trash is another man's treasure": what has often been described as "junk DNA" has revealed a hidden gem. Not only can we find the ancient ancestor of the human hepatitis B virus nestled in songbird genomes, but according to research published recently by a team of scientists at the University of Münster, this virus is 63 million years older than originally thought, a finding that may help improve human health outcomes.
Hepatitis B virus (HBV) is one of the most common human viral infections in the world. This virus specifically infects the liver cells of many primates (including humans), causing severe flu-like symptoms. Although most people fully recover, roughly 5 percent remain infected throughout their lives; acting as carriers who can infect others whilst also suffering a variety of serious liver diseases, including cancer. In fact, HBV is second only to tobacco amongst known human carcinogens, causing up to eighty percent of all hepatocellular carcinomas worldwide.
Where did HBV come from? Were other animals once infected by this virus before evolving defences against it? If so, how did these animals protect themselves from HBV?
Thanks to powerful new technologies, it now is possible to unearth the answers to these and other important questions by studying fossil virus DNA. Unlike most fossils, which leave either impressions or skeletal fragments in stone, viral fossils are remnants of ancient viral DNA trapped within the germlines of their hosts.
Although viral genomes evolve rapidly, their rate of change slows to the same pace as that of the host's DNA after insertion, making it possible to study viral DNA sequences that are many millions of years old.
"The prehistoric viral DNA becomes frozen in its original state at the time of insertion into the host genome and thus remains discernible as such until present", said Dr Jürgen Schmitz, a virologist at University of Münster and co-author on the paper.
By identifying, sequencing and analysing these genetic fingerprints, it is possible to reconstruct viral ancestors and learn more about them. This new field of study is known as paleovirology.

Zebra finch.
Building upon another group's earlier discovery that ancient HBV genomes had become trapped in the genomes of zebra finches (read more about that here), a team of scientists expanded upon that work. This team was led by Dr Alexander Suh, who conducted this work whilst a graduate student and postdoctoral fellow at the University of Münster in Germany.
In their work, Dr Suh and his colleagues sequenced and compared the viral fragments found in the genomes of a number of songbird species. They sifted through the avian DNA in search of HBV sequences, working back in evolutionary time from close relatives to zebra finches to more distantly related birds.
They found 16 viral fragments from HBVs that had been captured 12 different times in avian genomes, and eight of these were suitable for further analysis. Since the team could calculate how long ago these birds' common ancestors lived, they could use this information to pinpoint the age of each of these ancestral virus fragments residing in their genomes (figure 1; larger view):
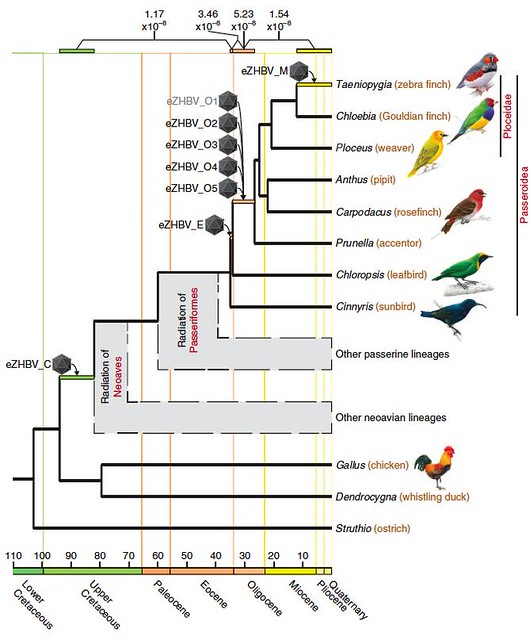 Figure 1 | Hepatitis B virus endogenization events during bird evolution. Endogenization events are represented with icosahedrons and temporal ranges of insertion events are shown as coloured rectangles. Figure 1 | Hepatitis B virus endogenization events during bird evolution. Endogenization events are represented with icosahedrons and temporal ranges of insertion events are shown as coloured rectangles.
[doi:10.1038/ncomms2798].
According to their findings, the first ancestral HBV became trapped in avian DNA quite early: the common ancestor of all the bird species carrying this particular viral fossil lived about 82 million years ago. This indicates that the ancestral HBV dates from the Late Mesozoic, shortly after the time when modern birds (neoaves) arose and before the songbird and parrot lineages split -- back when the dinosaurs were still very much alive.
But when did HBV begin infecting mammals? To answer this question, the team assembled two possible family trees for the hepadnaviruses (the HBV family), each one describing a different evolutionary scenario (figure 4; larger view):
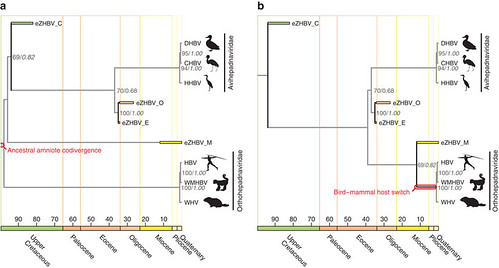 Figure 4 | Phylogeny of hepatitis B viruses and two scenarios on the origin of mammalian HBVs. [doi:10.1038/ncomms2798]. Figure 4 | Phylogeny of hepatitis B viruses and two scenarios on the origin of mammalian HBVs. [doi:10.1038/ncomms2798].
When the team rooted the hepadnaviral family tree to mammalian HBVs (figure 4a) -- a scenario suggesting that the common ancestor of this viral family may have first infected mammals -- their analysis showed that HBVs infected the first amniotes and split into separate lineages between 94 and 82 million years ago when the early bird and mammal lineages split.
Alternatively, when the hepadnaviral family tree was rooted to avian HBVs (figure 4b) -- a scenario suggesting that the common ancestor of hepadnaviruses may instead have first infected birds -- their analysis indicated that HBV began infecting mammals much later. This second scenario is more likely because of the apparent absence of any HBV fragments trapped in mammalian genomes and also because of the topology of the family tree.
"HBVs have probably been infecting mammals for a much shorter time than they have been infecting birds throughout much of their evolution," said Dr Suh in email.
"The most exciting and unexpected finding is that our oldest paleovirus is both extremely old and comprises a complete HBV genome sequence", said Dr Suh in email.
Nevertheless, despite its age, the reconstructed Mesozoic-era avian HBV is remarkably similar to the HBV that infects people today, the team found.
"We've had 82 million years of evolution, but they have the same proteins," said Dr Suh.
But the team's analysis showed one difference between the Mesozoic-era avian HBVs and the modern mammalian HBVs: the mammalian version manufactures one more protein, known as the X protein.
Originally, many scientists thought that avian HBVs lost the X protein during evolution, but the reconstructed ancestral HBVs didn't show any trace of the X protein, indicating that it was never present to begin with. The X protein is essential for HBV replication in mammals, indicating that gaining the ability to manufacture this protein was the reason that this HBV lineage switched hosts.
"The complete role of the X protein of mammalian HBVs is not known, but it has been shown to promote tumors", said Dr Suh in email.
These findings could enhance our understanding of HBV infectivity and improve health outcomes in humans.
"[F]uture biochemical analyses of the virus proteins (or even the in vitro resurrection of this paleovirus) could help us understand the evolution of host specificity in HBVs in general and the evolution of their (surface) proteins," said Dr Suh in email.
Paleoviruses are not limited to just birds. With the impending sequencing of many animal genomes, "we expect that our Mesozoic paleovirus genome is just the tip of the iceberg of prehistoric virus genomes" awaiting discovery, the team write in their paper.
Sources:Suh A., Brosius J., Schmitz J. & Kriegs J.O. (2013). The genome of a Mesozoic paleovirus reveals the evolution of hepatitis B viruses, Nature Communications, 4: 1791-1798. doi:10.1038/ncomms2798
Alex Suh, emails [30 April, 1 & 5 May 2013]
Universität Münster press release.
Image of a wild adult male zebra finch, Taeniopygia guttata, by [url=http://commons.wikimedia.org/wiki/User eripitus]Peripitus[/url]/Wikipedia, February 2009. [Multi-license with GFDL and Creative Commons CC-BY-SA-2.5 and older versions (2.0 and 1.0)] eripitus]Peripitus[/url]/Wikipedia, February 2009. [Multi-license with GFDL and Creative Commons CC-BY-SA-2.5 and older versions (2.0 and 1.0)]
Additional reading:
Warren W.C., Clayton D.F., Ellegren H., Arnold A.P., Hillier L.W., Künstner A., Searle S., White S., Vilella A.J. & Fairley S. & et al. (2010). The genome of a songbird, Nature, 464 (7289) 757-762. doi:10.1038/nature08819
Patel M.R., Emerman M. & Malik H.S. (2011). Paleovirology—ghosts and gifts of viruses past, Current Opinion in Virology, 1 (4): 304-309. doi:10.1016/j.coviro.2011.06.007
Gilbert C. & Feschotte C. (2010). Genomic Fossils Calibrate the Long-Term Evolution of Hepadnaviruses, PLoS Biology, 8 (9): e1000495. doi:10.1371/journal.pbio.1000495.s009
.. .. .. .. .. .. .. .. .. .. ..
Many thanks to my twitter pals, @bioSimonUoB, @Stephen_Curry & @McDawg, for emailing the requested PDFs necessary to research and write this story.
.. .. .. .. .. .. .. .. .. .. ..
|