|
  
- 现金
- 6115 元
- 精华
- 3
- 帖子
- 3925
- 注册时间
- 2001-12-21
- 最后登录
- 2019-5-28

|
2楼
 发表于 2008-5-24 19:37
发表于 2008-5-24 19:37
接上贴
Materials and Methods
Patients
Six human leucocyte antigen (HLA)-A2+ patients chronically infected with HBV were studied. The selection criteria were HBsAg and HBeAg positivity for at least 6 months, a liver biopsy within a year confirming chronic hepatitis with no evidence of cirrhosis, stable ALT values less than twice the upper limit of normal (i.e. <80 U/L) at least on two occasions 1 month before therapy and normal liver function (i.e. normal prothrombin time, serum albumin and bilirubin) ( Table 1 ). Screening for HLA-A2 positivity was performed by staining peripheral blood mononuclear cells (PBMC) of patients with fluorescent conjugated anti-HLA-A0201 antibody (Serotec, Kidlington, UK). All patients were negative for antibodies to hepatitis C virus, hepatitis D virus and human immunodeficiency virus (HIV)-1, 2. The longitudinal analysis of anti-viral T-cell immunity in the periphery was approved by the local ethics committee and all patients provided written consent.
Treatment
The six patients were treated with prednisolone, 30 mg/day for 2 weeks, tapering to 15 mg/day for a further 2 weeks, followed by 2 weeks without treatment. Lamivudine 100 mg/day was given from week 6 for 12 months (Fig. 1). Clinical, virological and immunological data were collected on two to three occasions preceding therapy, and over a period of 13 months (weeks −8, −4, 0, 4, 6, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48, 52 and 56).
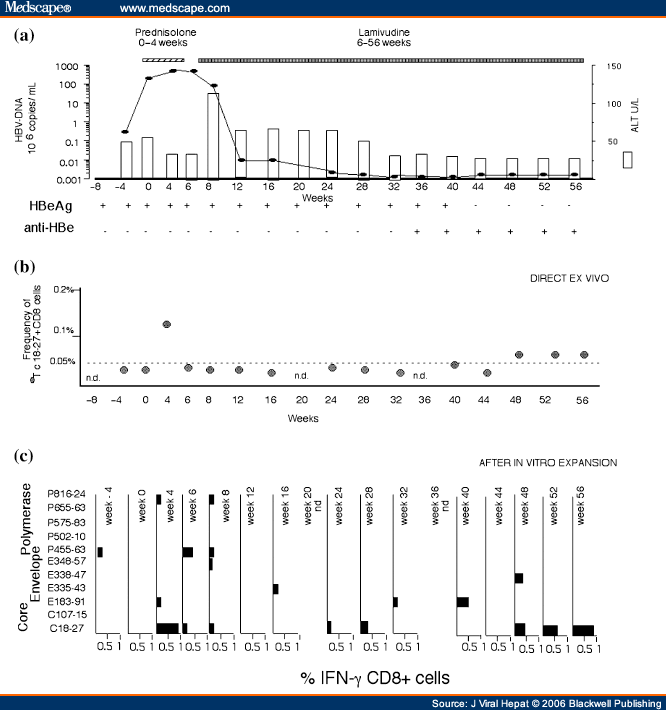 Click to zoom Click to zoom
Figure 1. (click image to zoom)
Longitudinal analysis of HBV-DNA levels, ALT and HBV-specific CD8+ T-cell responses in a treated representative patient. (a) Serum ALT levels (white bars) and HBV-DNA (black squares) are represented on the y-axis and measured at the indicated time points (x-axis). (b) Percentages of tetramer+ CD8+ T-cells are represented on the y-axis and the x-axis shows time in weeks prior to and during therapy. PBMC were tested directly ex vivo with the tetramers for core18-27, env183-91 and env348-57 specific CD8+ T-cells. Positive results were detected only using tetramer core18-27 (Tc18-27 •). The background level of direct tetramer staining with Tc18-27 (0.05%, indicated by a horizontal line) was calculated in HLA-A2 + non-HBV-infected subjects and in HLA-A2-negative HBV-infected patients.[9,29] (c) Percentage of peptide-specific IFN-γ-producing CD8+ T-cells after in vitro expansion (bars). PBMC were stimulated with the indicated peptides. After 10 days of in vitro expansion, the frequency of peptide-specific IFN-γ-producing CD8+ T-cells was calculated. The indicated percentages of IFN-γ-producing CD8+ T-cells were calculated after subtraction of double positive cells present in non-peptide stimulated cells.
Virological Assessment
Hepatitis B surface antigen, anti-HBs, total and IgM anti-HBc, HBeAg, anti-HBe, were determined by commercial enzyme immunoassay kits (Abbot Laboratories, North Chicago, IL, USA; Ortho Diagnostic System, Raritan, NJ, USA; Sanofi Diagnostic Pasteur, Marnes-la Coquette, France). Serum HBV-DNA was quantified using the Roche Amplicor Monitor assay (Roche Pharmaceuticals Ltd., Branchburg, NJ, USA), with a DNA detection limit of 400 copies/mL (0.0014 pg/mL).
PCR and HBV-DNA Sequencing
DNA was extracted from serum samples using the QIAamp DNA Blood mini kit (QIAGEN, Crawley, UK). The HBV-DNA was amplified with primers specific for the HBV core and envelope genes, as described previously.[24] The amplicons were purified and the core/envelope regions were sequenced directly using an ABI 377 Automated Sequencer (Applied Biosystems, Foster City, CA, USA). The HBV genotype infecting these patients was determined by analysis of sequenced portions of the core and surface antigens.[14]
Synthetic Peptides
Peptides corresponding to different HBV-specific HLA-A2 restricted CD8 epitopes were purchased from Primm (Milan, Italy). The purity of peptides was greater than 90% by high-performance liquid chromatography (HPLC) analysis. The peptides were based on HBV genotype D (serotype ayw) and the degree of homology among different genotypes is shown in Table 2 .
Isolation of PBMCs and Production of T-cell Lines
Peripheral blood mononuclear cells were isolated from fresh heparinized blood by Ficoll-Hypaque density-gradient centrifugation and suspended at a concentration 1.5-2 × 106 cells/mL in RPMI1640 (Invitrogen Ltd., Paisley, UK), 10% foetal calf serum (FCS). Cells were stimulated with 1 μm of various peptides in a 96-well plate. Recombinant interleukin 2 (IL-2) (R&D systems, Abingdon, UK) was added on day 4 of culture and cells were analysed after 10-12 days of culture. PBMC stimulation was performed with a panel of 11 peptides ( Table 2 ) corresponding to HBV sequences previously identified as HLA-A2-restricted CTL epitopes.[15]
Intracellular IFN-γ Production
Ex vivo-purified PBMC or short-term T-cell lines were stimulated at 2-3 × 106 cells/mL in RPMI1640, 10% FCS, with HBV peptides (1 μm) for 6 h at 37 °C in the presence of Brefeldin A (Sigma-Aldrich, Poole, Dorset, UK) at 10 μg/mL. Cells were washed, stained with Cy-chrome-conjugated anti-CD8 antibodies, then permeabilized and fixed using Cytofix/Cytoperm (Pharmingen, San Diego, CA, USA) according to the manufacturer's instructions. Flourescein isothiocyanate (FITC)-conjugated anti-cytokine antibodies or isotype-matched controls were added (30 min, 22 °C), washed twice and analysed by flow cytometry.
Staining With HLA-tetrameric Complexes
Human leucocyte antigen-class I tetramers were purchased commercially (Proimmune, Oxford, UK). Tetramer staining was performed as previously described[6] and cells were analysed on FACSort® (Becton Dickinson: BD Biosciences, San Diego, CA, USA) using CELL Quest™ (BD Biosciences, San Diego, CA, USA) software immediately or after addition of 1% paraformaldehyde.
[ 本帖最后由 FORRESTFU 于 2008-5-24 19:39 编辑 ] |
|
 发表于 2008-5-24 19:35
发表于 2008-5-24 19:35
 发表于 2008-5-24 19:37
发表于 2008-5-24 19:37
 发表于 2008-5-24 19:43
发表于 2008-5-24 19:43
 发表于 2008-5-24 19:50
发表于 2008-5-24 19:50
 发表于 2008-5-24 19:55
发表于 2008-5-24 19:55
 发表于 2008-5-25 08:52
发表于 2008-5-25 08:52
 发表于 2008-5-25 10:43
发表于 2008-5-25 10:43