 |  |  |
| Phase 2a, Randomized, Double-blind, Placebo-controlled Study of an Antisense Inhibitor (ISIS 505358)in Treatment-naïve Chronic Hepatitis B (CHB) Patients: Safety and Antiviral Efficacy
|
|
| AASLD 2019 Nov 8-11 Boston
Man Fung Yuen1 Jeong Heo2, Jeong Won Jang3, Jung-Hwan Yoon4, Young-Oh Kweon5, Sung Jae Park6, C. Frank Bennett7, and T. Jesse Kwoh71Queen Mary Hospital, The University of Hong Kong, Hong Kong, 2Pusan National University and Medical Research Institute, Pusan National University Hospital, Busan, Republic of Korea, 3Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Republic of Korea,4Seoul National University Hospital, Seoul, Republic of Korea, 5Kyungpook National University Hospital, Daegu, Republic of Korea, 6Inje University Busan Paik Hospital, Busan, Republic of Korea, and 7Ionis Pharmaceuticals Inc., Carlsbad, CA
GSK is moving forward developing ISIS 50538, an unconjugated ASO.Ionis announced in a press release August 27, 2019 that GSK exercised its option to license Ionis' antisense HBV program. Going forward the unconjugated ASO will use the GSK compound number GSK3228836 
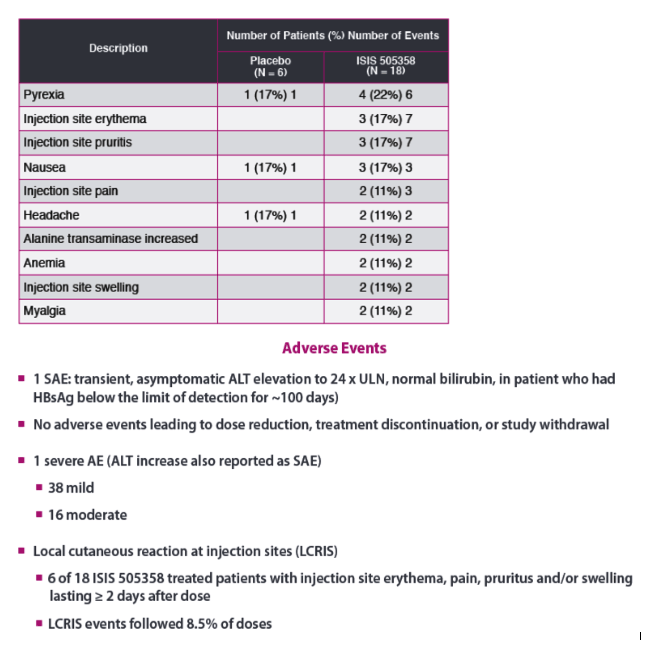
|
|
|  |  |
|
|