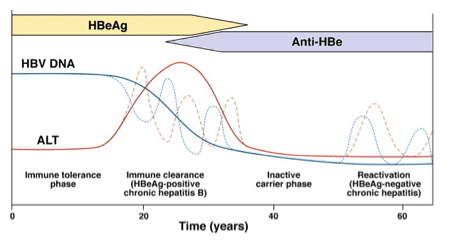
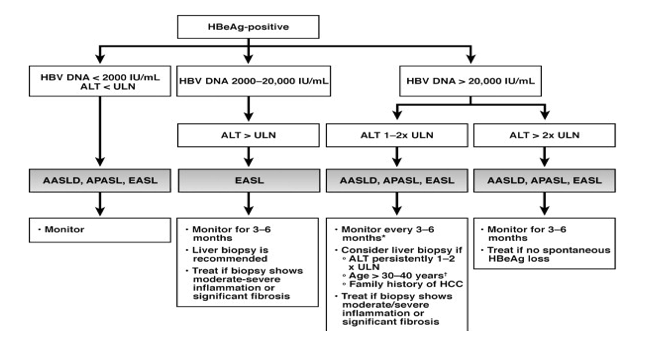
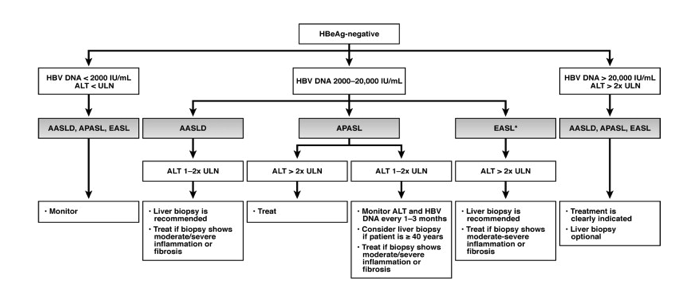
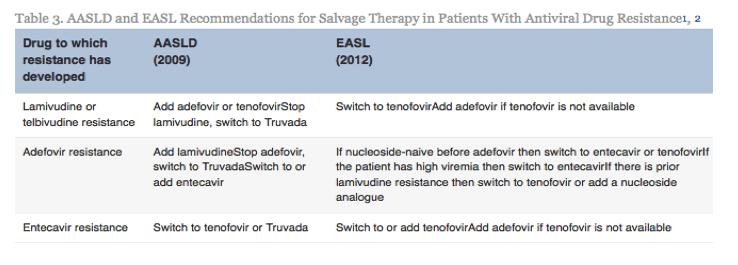
| Our Practice Quantitative HBsAg assays are not available for clinical use in the United States. We recommend completion of the intended duration of PEG-IFN therapy unless the patient experiences serious adverse events or there is little or no decrease in serum HBV DNA level after 3 to 6 months treatment. For NUC-naive patients receiving entecavir or tenofovir, we have encountered only 1 patient (out of >200) with confirmed entecavir resistance and none with confirmed tenofovir resistance. We found that transient reappearance of serum HBV DNA at low levels, typically less than 100 IU/mL, occurs in some patients. Although many of these instances may be related to medication nonadherence, some, particularly those with levels below the limit of quantification, may represent false-positive results. In NUC-naive patients receiving entecavir or tenofovir monotherapy with detectable HBV DNA levels after 1 year of treatment, we have not adapted treatment as long as the HBV DNA level is low (<10,000 IU/mL) and continues to decrease. We have added a second drug in a few patients on dialysis receiving weekly dosing of entecavir and 2 patients with high baseline HBV DNA levels receiving immunosuppressive therapy. 我们的实践 HBsAg定量测定法不适用于在美国的临床使用。我们推荐的PEG- IFN治疗的预期持续时间的结束,除非患者经历严重的不良事件或存在于血清中的HBV DNA水平很少或不下降后3至6个月的治疗。 对于接受恩替卡韦或替诺福韦NUC初治患者,我们只遇到1例(满分> 200)证实恩替卡韦耐药,没有确诊替诺福韦阻力。我们发现,血清HBV DNA在较低水平,典型地小于100国际单位/毫升,瞬态再现发生在一些患者中。虽然许多这些实例可能与药物的不依从,一些,特别是那些低于定量限电平,可以表示假阳性结果。在治疗1年后接受恩替卡韦或替诺福韦单药治疗与检测HBV DNA水平NUC初治患者,我们没有,只要HBV DNA水平低( < 10,000国际单位/毫升),并继续降低适应的待遇。我们已经在少数透析患者接受恩替卡韦和2例高基线HBV DNA水平接受免疫抑制剂治疗的剂量每周增加了第二种药物。 Conclusions Guidelines provide an evidence-based framework for managing patients; however, management of individual patients must be flexible, taking into account the patient's preference and other medical or psychosocial conditions, evolution in knowledge over time, and the provider's experience. 结论 指南提供以证据为基础的框架,用于管理病人,但是,个别病人管理必须是灵活的,考虑到病人的偏好和其他医疗或心理状况,演变知识随着时间的推移,与供应商的经验 | ||
| 欢迎光临 肝胆相照论坛 (http://hbvhbv.info/forum/) | Powered by Discuz! X1.5 |